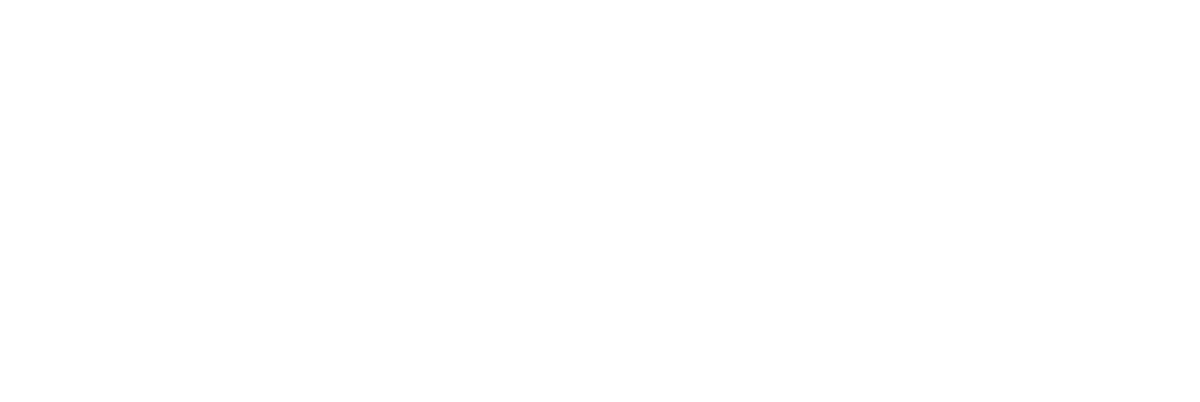A Catalytic One-Pot Synthesis of Indolyl Cyclobutanones
Stefania Porcu, Carla Aira Rodriguez, Angelo Frongia, Francesco Secci Synthesis, 2021 feature article
DOI: 10.1055/s-0040-1706087

A general strategy for the synthesis of indolyl cyclobutanones via a tandem Brønsted acid catalyzed 2-hydroxycyclobutanone activation–indole nucleophilic addition has been exploited. The procedure leads to a wide range of 2- and 3-functionalized indole derivatives in good to high yields with broad substrate scope.
Visible light promoted continuous flow photocyclization of 1,2-diketones
Francesco Secci, Stefania Porcu, Alberto Luridiana, Angelo Frongia, Pier Carlo Ricci Org. Biomol. Chem., 2020,18, 3684-3689
https://pubs.rsc.org/en/content/articlelanding/2020/OB/D0OB00532K#!divAbstract
The continuous flow Norrish–Yang photocyclization of 1,2-diketones has been developed and used for the synthesis of a large number of functionalized 2-hydroxycyclobutanones, under blue light irradiation and employing acetone as a solvent. This eco-friendly procedure represents a valid alternative to the reactions carried out in batches thus reducing the reaction times, the formation of secondary products and simplifying the purification steps. The use of differently substituted diketone compounds has allowed us to obtain a wide range of 2 and 3-functionalized cyclobutanones, thus allowing the evaluation of the scope and limitations of this procedure.
Catalytic Tandem Friedel–Crafts Alkylation/C4–C3 Ring-Contraction Reaction: An Efficient Route for the Synthesis of Indolyl Cyclopropanecarbaldehydes and Ketones
Francesca Turnu, Alberto Luridiana, Andrea Cocco, Stefania Porcu, Angelo Frongia, Giorgia Sarais, Francesco Secci Org. Lett. 2019, 21, 18, 7329–7332
https://pubs.acs.org/doi/10.1021/acs.orglett.9b02617
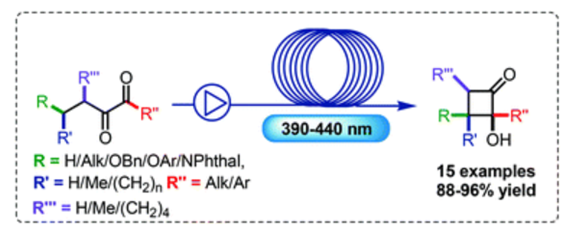
A general strategy for the synthesis of indolyl cyclopropanecarbaldehydes and ketones via a Brønsted acid-catalyzed indole nucleophilic addition/ring-contraction reaction sequence has been exploited. The procedure leads to a wide panel of cyclopropyl carbonyl compounds in generally high yields with a broad substrate scope
Brønsted Acid Mediated Cascade Reaction To Access 3-(2-Bromoethyl)benzofurans
Stefania Porcu, Stefania Demuro, Alberto Luridiana, Andrea Cocco, Angelo Frongia, David J. Aitken, Florence Charnay-Pouget, Regis Guillot, Giorgia Sarais, Francesco Secci Org. Lett. 2018, 20, 23, 7699–7702
https://pubs.acs.org/doi/10.1021/acs.orglett.8b03429
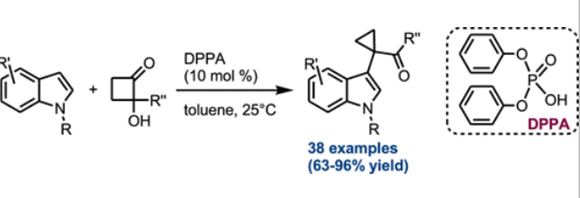
A unified protocol for the construction of 3-(2 bromoethyl)benzofurans and 2-(benzofuran-3-yl)ethylamines from bis[(trimethylsilyl)oxy]cyclobutene has been developed. This mild and facile strategy was applied for the synthesis of a series of 5-HT serotonin receptor agonists, underlining its potential for the syntheses of bioactive compounds and natural products
Acid-catalyzed synthesis of functionalized arylthio cyclopropane carbaldehydes and ketones
Stefania Porcu, Alberto Luridiana, Alberto Martis, Angelo Frongia, Giorgia Sarais, David J. Aitken, Thomas Boddaert, Regis Guillot, Francesco Secci Chem. Commun., 2018,54, 13547-13550
https://pubs.rsc.org/en/content/articlelanding/2018/CC/C8CC07571A#!divAbstract

A general strategy for the synthesis of arylthio cyclopropyl carbaldehydes and ketones via a Brønsted acid catalyzed arylthiol addition/ring contraction reaction sequence has been exploited. The procedure led to a wide panel of cyclopropyl carbaldehydes in generally high yields and with broad substrate scope. Mechanistic aspects and synthetic applications of this procedure were investigated.
A catalyst-free, waste-less ethanol-based solvothermal synthesis of amides
Francesca Dalu, Mariano A. Scorciapino, Claudio Cara, Alberto Luridiana, Anna Musinu, Mariano Casu, Francesco Secci, Carla Cannas Green Chem., 2018,20, 375-381
https://pubs.rsc.org/en/content/articlelanding/2018/GC/C7GC02967E#!divAbstract
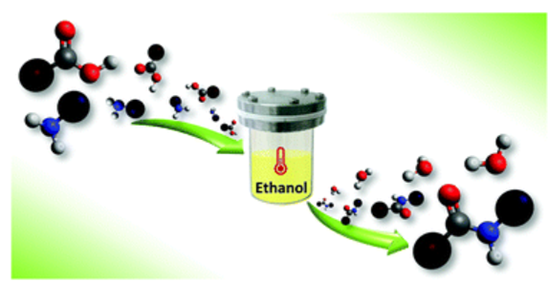
A green, one-pot approach based on the solvothermal amidation of carboxylic acids with amines has been developed for the synthesis of diverse aliphatic and aromatic amides. It does not require the use of catalysts or coupling reagents and it occurs in the presence of ethanol that has been proved to have a key role in the process. The proposed strategy is also extendable to biologically active amides and could represent a low-cost and waste-less alternative to the common synthetic pathways.
Acid-catalyzed reaction of 2-hydroxycyclobutanone with benzylic alcohols
Alberto Martis, Alberto Luridiana, Angelo Frongia, Massimiliano Arca, Giorgia Sarais, David J. Aitken, Regis Guillot, Francesco Secci Org. Biomol. Chem., 2017,15, 10053-10063
https://pubs.rsc.org/en/content/articlelanding/2017/OB/C7OB02545A#!divAbstract
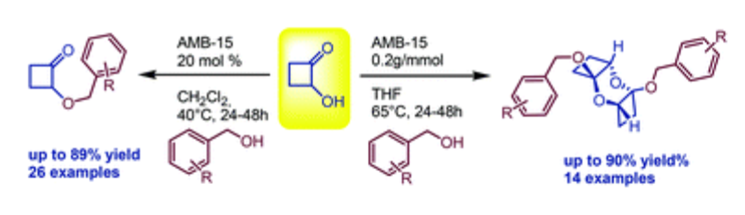
The acid-promoted syntheses of 2-(benzyloxy)cyclobutanones and bis(benzyloxy)dioxatricyclo decanes were achieved starting from 2-hydroxycyclobutanone and variously functionalized benzyl alcohols. The reaction sequences afforded the desired products in good to high yields and in a solvent-dependent chemoselective fashion.
Deracemizing organocatalyzed Michael addition reactions of 2-(arylthio)cyclobutanones with β-nitrostyrenes
Alberto Luridiana, Angelo Frongia, David J. Aitken, Regis Guillot, Giorgia Sarais, Francesco Secci Org. Biomol. Chem., 2016,14, 3394-3403
https://pubs.rsc.org/en/content/articlelanding/2016/OB/C6OB00160B#!divAbstract
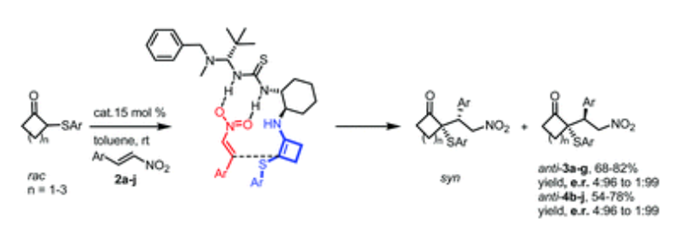
Organocatalyzed Michael addition reactions of 2-(arylthio)cyclobutanones with trans-β-nitrostyrenes have been carried out using a bifunctional thiourea-primary amine catalyst, providing diastereoisomerically and enantiomerically enriched 2-alkyl-2-(arylthio)cyclobutanones having two contiguous stereocenters of which one is a chiral quaternary center. The absolute configuration of these novel adducts was assigned by X-ray diffraction analysis and a transition-state model is proposed to explain the observed stereoselectivities
Disulfide-based metal-free α-sulfanylation of ketone
Andrea F. Vaquer, Angelo Frongia, Francesco Secci, Enrica Tuveri
RSC Adv., 2015,5, 96695-96704
https://pubs.rsc.org/en/content/articlelanding/2015/RA/C5RA17913K#!divAbstract
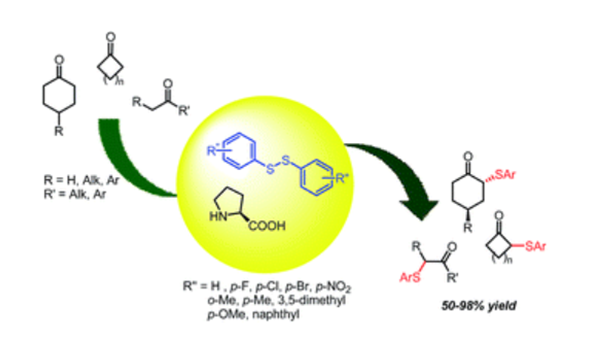
An eco-friendly methodology for the direct α-sulfanylation of ketones, has been developed. The procedure, based on the use of functionalized diaryldisulfides and catalyzed by D,L-proline, represents a mild and efficient approach for the preparation of α-arylthio-ketones.
Enantioselective Organocatalyzed Desymmetrization of 3-Substituted Cyclobutanones through Michael Addition to Nitroalkenes
Francesca Capita, Angelo Frongia, Jean Ollivier, David J. Aitken, Francesco Secci, Pier Paolo Piras, Regis Guillot
Synlett 2015; 26, 123-126
DOI: 10.1055/s-0034-1379489
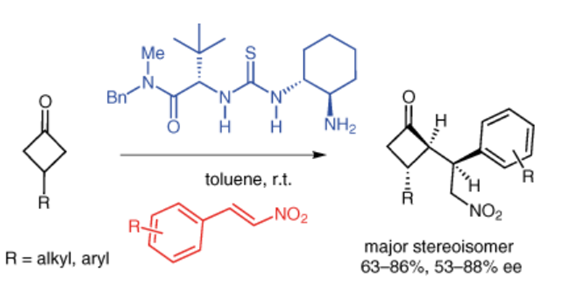
A new procedure for the desymmetrization of prochiral 3-substituted cyclobutanones has been established through organocatalyzed Michael addition to nitroalkenes. The approach provides enantiomerically enriched 2-alkyl-3-aryl(alkyl) cyclobutanones with three contiguous stereogenic centers. The optimum conditions were determined by screening of catalyst and reaction conditions and a transition-state model is proposed to account for the observed diastereomeric and enantiomeric selectivities.
Tetrazole amides as hydrogen-bonding donor catalysts in the chemoselective oxidation of sulphides and disulphides
Francesco Secci, Massimiliano Arca, Angelo Frongia, Pier Paolo Piras Catal. Sci. Technol., 2014,4, 1407-1415
https://pubs.rsc.org/en/content/articlelanding/2014/CY/c4cy00086b#!divAbstract

The oxidation of organosulphides catalyzed by hydrogen bonding donors deriving from aminotetrazole has been studied. The oxidation reaction was performed in CH2Cl2 solution using TBHP (1.1 eq.) as a versatile and chemoselective new catalyst to sulfoxides. 5 mol.% catalyst loading afforded organosulfoxides with complete conversion and yields around 90–95%. Tetrazole amide derivatives can be easily recovered by simple filtration and reused several times. Reactions were carried out in scales ranging between mg and multigram in order to test the robustness of the process. 1H-NMR studies and DFT calculations were exploited to disclose the role of tetrazole amide–TBHP complexes in the sulphides oxidation reaction as new performing catalysts.
New aminotetrazole derivatives as hydrogen bonding catalysts. A green and selective oxidation of organosulphides with H2O2 in H2O
Francesco Secci, Massimiliano Arca, Angelo Frongia, Pier Paolo Piras New J. Chem., 2014,38, 3622-3629
https://pubs.rsc.org/en/content/articlelanding/2014/NJ/C4NJ00530A#!divAbstract

The oxidation of organosulphides catalysed by hydrogen bonding donors derived from aminotetrazole has been studied. The oxidation reaction was performed in a H2O solution using H2O2 as a versatile, green and chemoselective new approach to sulphoxides. Sulphoxide compounds are obtained in high yields and excellent selectivity through a new and easy to perform oxidation protocol. Aminotetrazole derivatives can be recycled by filtration and reused several times without expensive purification procedures.
From (Phenylsulfanyl)cycloalkanecarbaldehydes to Optically Active Spirocyclic Tetrahydrofurans: Stereospecific Resolution of Symmetric Aldehydes through (S)‐Proline‐Catalysed Aldol Reaction
Francesco Secci, Angelo Frongia, Maria Grazia Rubanu, Maria Laura Sechi, Giorgia Sarais, Massimiliano Arca, Pier Paolo Piras Eur J. Org. Chem. 2014, 6659-6675
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/ejoc.201402653

A variety of enantiomerically enriched tetrahydrofuran spirocyclic derivatives have been synthesised by a sequential enantioselective proline‐induced stereospecific resolution through aldol reaction of (E/Z)‐(phenylsulfanyl)cycloalkanecarbaldehydes followed by a ketone reduction, acid‐catalysed [1,2]‐SPh stereospecific migration, and ring‐closure reaction.